RA History: What have we learned in the last 5 decades? Adjuvants in Regional Anesthesia: Lessons Learned
Adjuvants in Regional Anesthesia: Lessons Learned.
Andre VAN ZUNDERT (1), Kai WOODFALL (2), Shady EKLADIOUS (3), Robert NICOLAE (3)
1. Department of Anaesthesia & Perioperative Medicine, Royal Brisbane and Women’s Hospital & The University of Queensland, Herston-Brisbane, Australia
2. Department of Anaesthesia & Perioperative Medicine, Royal Brisbane & Women’s Hospital & The University of Queensland, Brisbane, Australia, none, Brisbane, Australia
3. Department of Anaesthesia & Perioperative Medicine, Royal Brisbane & Women’s Hospital & The University of Queensland, Brisbane, Australia, none, Brisbane, QLD, Australia
Key Reasons why Regional Anesthesia is Preferred by Patients, Surgeons and Anesthesiologists
The choice of anesthesia must be tailored to each patient’s specific circumstances and the type of surgery. However, regional anesthesia (RA) offers numerous benefits over general anesthesia (GA) for many surgical patients and has been advocated as a valuable adjunct to a multimodal analgesic regimen. These benefits span across overall experience and patient safety, i.e., improved pain management, higher patient comfort and satisfaction, faster recovery, reduced systemic side effects, and fewer respiratory and cardiovascular complications.
In terms of environmental impact, RA has several benefits compared to GA: a) complete avoidance of potent anesthetic greenhouse gases results in a decreased atmospheric pollution, a smaller carbon footprint, and reduced long-term pollution; b) lower energy consumption as the patient is in the operating room for a shorter time, requires less monitoring and less electrically-operated medical equipment, and reduces the need for intense ventilation to clear anesthetic gases, which itself engenders significant energy use; c) RA generates less disposable consumption, leading to less medical waste; d) localized delivery of anesthetic agents reduces the overall quantity of pharmaceuticals entering the environment through patient excretion and drug wastage; e) some equipment used in RA, i.e., nerve stimulators, ultrasound devices, is reusable and has a longer lifespan compared to the single use items often used for GA. One potential environmental downside for RA compared to GA is an increased burden of sterilization and thus electrical consumption. However, this is unlikely to offset the overall environmental benefit of RA.
Some of the key reasons why RA is often preferred include: a) improved pain management: RA provides targeted pain relief at the surgical site, leaving the areas above and below surgery unaffected. Perioperative pain relief is often superior by effectively controlling pain with local anesthetics (LAs), decreasing the need for systemic opioids, thereby lowering the risk of opioid-related side effects and dependency; b) enhanced recovery and mobility: Patients often recover more quickly from RA, experiencing less grogginess and confusion compared to those recovering from GA. This facilitates earlier postoperative mobilization, which is crucial for reducing risks of complications (e.g., deep vein thrombosis) and promoting faster overall recovery; c) fewer respiratory complications due to the avoidance of airway manipulation and preservation of respiratory function: GA requires airway management with its inherent complications (sore throat, hoarseness, and in severe cases, aspiration or respiratory distress). RA avoids these risks by eliminating the need for intubation, preserving the patient’s respiratory function, allowing spontaneous breathing. RA is particularly beneficial for those with existing respiratory conditions; d) cardiovascular stability due to reduced hemodynamic fluctuation: RA typically results in more stable blood pressure and heart rate compared to the hemodynamic changes that can occur with the induction and emergence phases of GA. For patients with cardiovascular conditions, the reduced stress on the heart makes RA a safer option; e) reduced systematic side effects due to the minimized drug exposure and lowered risk of cognitive dysfunction: RA involves less exposure to systemic medications, reducing the risk of drug-related side effects, i.e., nausea and vomiting, and respiratory depression. GA can lead to postoperative cognitive delirium and dysfunction, particularly in the elderly population. RA reduces this risk by avoiding systemic sedatives and anesthetics that affect the brain; f) higher overall patient satisfaction and comfort: RA allows the patients to remain awake or lightly sedated during surgery, allowing them to avoid the disorienting effects of GA; g) RA may not be suitable for all types of surgeries or all types of patients. However, for many complex surgeries that typically require GA, RA can complement by providing excellent pain relief; and h) RA is cost-effective: RA allows faster recovery times which can lead to shorter hospital stays, which is cost-effective for healthcare and the patient. The decreased use of systemic anesthetics and opioids may lower the overall cost of medications.
Whether LAs provide the best perioperative analgesia depends on various factors, including the type of surgery, patient characteristics, and the desired outcomes. LAs provide targeted pain relief by blocking nerve signals in the area of administration, which can be very effective for many surgical procedures using central neuraxial and peripheral nerve blockade, with long-lasting pain relief if catheters are used, often combined with continuous infusion pumps. LAs can be used in various forms, including topical applications, infiltration blockade, nerve blocks, and spinal, epidural or combined anesthesia techniques, making them versatile for different surgical needs.
Limitations of LAs
LAs are highly effective for perioperative pain management, providing targeted analgesia with minimal systemic side effects. However, there are limitations using solely LAs: a) insufficient duration of action: the analgesic effect of LAs is limited to the duration of the block, which may not cover the entire perioperative period. Longer-acting agents, continuous infusion techniques and the use of adjuvants can mitigate these limitations but will add to the complexity of blocks; b) incomplete RA blockade requires supplemental pain management strategies; c) LAs are generally safe but still can cause complications such as LA systemic toxicity (LAST) due to massive resorption or intravascular injections, allergic reactions, or damage to muscles (LA-induced myotoxicity and myo-degeneration), nerves, or spinal cord if improperly administered or when high doses are used.1
However, their limitations in duration and potential for incomplete pain relief make them most effective when used as part of a multimodal pain management strategy. By combining LAs with other analgesics, adjuvants, and techniques, anesthesiologists can achieve optimal pain control tailored to individual patient needs and surgical contexts.
Adjuvants to LAs
As the indications for RA have gradually expanded, adjuvants are frequently incorporated to enhance patient safety and comfort, improve efficacy, onset, quality and duration of analgesia, reduce the required dose of LAs and minimize potential side effects.2-6
The benefits of these adjuvants include a faster onset of block, improved hemodynamic stability, reduced postoperative opioid requirements, anti-inflammatory effects, and additional anxiolysis and sedation. These advantages contribute to better pain management, increased patient satisfaction, enhanced clinical outcomes, and improved overall perioperative results. These substances can be added to LAs for various types of regional blocks, including peripheral nerve blocks, fascia blocks, central neuraxial blocks, ophthalmic blocks, and intravenous RA blocks, with the intention of blocking transmission to avoid or relieve pain. Anesthesiologists select these adjuvants based on specific clinical scenarios and surgical interventions, patient-specific factors, type of RA, desired effects, and a balance of their benefits against potential side effects.
Table 1 provides an unrestricted list of potential useful adjuvants to LAs for a variety of RA blocks, including central neuraxial and peripheral nerve blocks. Suggested doses are provided, though clinicians need to verify each dosage according to their local circumstances, the surgical intervention, and the individual patient. LAs and adjuvants are used in a large range of medication types, volumes, doses, and concentrations. It is crucial to consider the appropriate drug in the right volume/concentration/dose for each specific RA technique. Clinicians should evaluate all substances in the correct LA solution for the right indication before any injection. Not all RA adjuvants have been approved by the regulators or licensed for neuraxial administration in all countries, and some preparations may contain additives, such as preservatives that are potentially neurotoxic. In specific clinical circumstances (e.g., existing diabetic neuropathy) some practice modifications may be considered to reduce the risk of overdose, side effects and complications.7 Clinicians need to be diligent about monitoring for the development of adverse side effects and complications from LAs and RA adjuvants and their immediate appropriate management. These common side effects limit their clinical use and may pose an even greater threat in certain procedures, including organ damage.
Opioids act as agonists at G-protein coupled inhibitory receptors, i.e., mu, kappa, delta, and nociceptin. These opioid receptors are widespread throughout the brain (cerebrum, thalamus, hypothalamus, amygdalae, basal ganglia, brainstem, reticular activating system), spinal cord, and non-neural tissues (gastrointestinal tract). Side effects often seen following neuraxial administration of opioids due to their cephalad spread in the CSF or systemic absorption from the epidural space, include pruritus, PONV, urinary retention, and respiratory depression. Minute doses of fentanyl or sufentanil are useful adjuvants to low-dose LAs.8,9
Adverse effects following the administration of LA mixtures are a concern, including cardiopulmonary, neurological, and renal complications, as well as uncommon reactions such as allergy and rarely malignant hyperthermia. Adjuvants to LAs have their own side effects (see Table 1). Therefore, further research on the development of novel LA adjuvants is necessary.
Liposomal bupivacaine is an example of an extended-release formulation that allows for a slow release of bupivacaine HCl from its liposomes. Another promising avenue is the use of exosomes, a class of new bioactive substances released from specific cells, which show unique effects in repairing damaged tissues and organs.10Exosomes released from cardiomyocytes after exercise have powerful cardioprotective effects, while those released from mesenchymal stem cells can improve neural cell damage. Exosomes originating from the cerebrospinal fluid can promote neuronal repair processes. Exosomes may help to overcome the hazards of LA adjuvants, such as cardiovascular, neurotoxic and gastrointestinal risks. Animal research has demonstrated that exosomes derived from different tissue cell sources exhibit repair functions after ischemia-reperfusion injuries, causing cellular metabolic acidosis and short-term organ damage. Exosomes released by specific cell types have been found to exert similar effects as many LA adjuvants. Therefore, these exosomal anesthetic adjuvants can be considered as novel LA adjuvant drugs with additional organ repair functions due to their reduction of the inflammatory response and pain relief. Exosomes exhibit reno-, neuro- and cardioprotective effects and immunosuppressive effects similar to those of stem cells. Reduction of postoperative pain is associated with exosomes of macrophage origin.
There are numerous aspects of RA adjuvants that were not addressed in this manuscript: a) all adjuvants available for use during RA, i.e., neostigmine and non-steroid anti-inflammatory drugs; b) alternative locally administered analgesic agents that have local anesthetic properties, e.g., tramadol; c) the efficacy of perioperative gabapentin in the treatment of postoperative pain; d) which adjuvants are preferred for specific circumstances e.g., which opioid is superior for a specific RA block; e) adjuvants that are better avoided due to their potential for adverse effects, limited efficacy, or safety concerns, e.g., vasopressin; f) the maximum dosage used in the different blocks; g) the potential of novel local anesthetics with protracted analgesic effect and minimal toxicity, which are neurotoxins isolated from animals, plants, and marine organisms, e.g., α-cobratoxin (α-CTx). The latter is isolated from the Thailand Cobra, which has strong affinity for the α7 subunit of the nAChR (α7nAChR) neuronal receptor of the peripheral nervous system. This neurotoxin leads to the depolarization of postsynaptic membranes and the prevention of neurotransmitter release, hence causing pain relief.
Conclusion
Medications used in anesthesia represent one of the greatest discoveries in medical history, revolutionizing pain management and patient care. The indications for LAs and RA blocks have gradually expanded, often in combination with general anesthesia. When used appropriately, adjuvants can significantly enhance the efficacy of RA, though potential adverse reactions must be carefully managed. These additives may improve the RA block’s quality, onset time, duration, or performance (such as motor blockade). Drugs utilized during RA procedures play a crucial role in perioperative pain prevention and relief.
The growing interest in RA techniques has spurred efforts to extend the duration of LAs. The development of LA adjuvants has been instrumental in mitigating the side effects and complications associated with large doses of LAs, including systemic and neurotoxicity risks. These adjuvants have effectively reduced LA toxicity, improved patient satisfaction, and decreased pain experiences. Adjuvants have also enhanced the speed of recovery, facilitated operator convenience, reduced postoperative delirium and increased the efficiency and safety of RA procedures. Continued research and innovation in new LA adjuvants will further advance the field of anesthesia, offering safer and more effective pain management solutions.
References
1. Hussain N, McCartney CJL, Neal JM, Chippor J, Banfield L, Abdallah FW. Local anaesthetic-induced myotoxicity in regional anaesthesia: a systematic review and empirical analysis. Br J Anaesth. 2018 Oct;121(4):822-841. doi: 10.1016/j.bja.2018.05.076. PMID: 30236244.
2. Bao N, Shi K, Wu Y, He Y, Chen Z, Gao Y, Xia Y, Papadimos TJ, Wang Q, Zhou R. Dexmedetomidine prolongs the duration of local anesthetics when used as an adjuvant through both perineural and systemic mechanisms: a prospective randomized double-blinded trial. BMC Anesthesiol. 2022 Jun 7;22(1):176. doi: 10.1186/s12871-022-01716-3. PMID: 35672660; PMCID: PMC9172023.
3. Martin MTF, Alvarez Lopez S, Aldecoa Alvarez-Santullano C. Role of adjuvants in regional anesthesia: A systematic review. Rev Esp Anestesiol Reanim (Engl Ed). 2023 Feb;70(2):97-107. doi: 10.1016/j.redare.2021.06.006. PMID: 36813032.
4. Coppens SJR, Zawodny Z, Dewinter G, Neyrinck A, Balocco AL, Rex S. In search of the Holy Grail: Poisons and extended release local anesthetics. Best Pract Res Clin Anaesthesiol. 2019 Mar;33(1):3-21. doi: 10.1016/j.bpa.2019.03.002. PMID: 31272651.
5. Prabhakar A, Lambert T, Kaye RJ, Gaignard SM, Ragusa J, Wheat S, Moll V, Cornett EM, Urman RD, Kaye AD. Adjuvants in clinical regional anesthesia practice: A comprehensive review. Best Pract Res Clin Anaesthesiol. 2019 Dec;33(4):415-423. doi: 10.1016/j.bpa.2019.06.001. Erratum in: Best Pract Res Clin Anaesthesiol. 2021 Dec;35(4):E3-E4. doi: 10.1016/j.bpa.2020.09.002. PMID: 31791560.
6. Tresierra S, Gilron I, Mizubuti GB. Adjuvant Medications for Peripheral Nerve Blocks. ATOTW 489, 2023. https://resources.wfsahq.org/anaesthesia-tutorial-of-the-week/
7. Lirk P, Brummett CM. Regional anaesthesia, diabetic neuropathy, and dexmedetomidine: a neurotoxic combination? Br J Anaesth. 2019 Jan;122(1):16-18. doi: 10.1016/j.bja.2018.09.017. PMID: 30579401.
8. Dong J, Jin Z, Chen H, Bao N, Xia F. Sufentanil Improves the Analgesia Effect of Continuous Femoral Nerve Block After Total Knee Arthroplasty. J Pain Res. 2023 Dec 7;16:4209-4216. doi: 10.2147/JPR.S409668. PMID: 38090025; PMCID: PMC10712246.
9. Kim SY, Cho JE, Hong JY, Koo BN, Kim JM, Kil HK. Comparison of intrathecal fentanyl and sufentanil in low-dose dilute bupivacaine spinal anaesthesia for transurethral prostatectomy. Br J Anaesth. 2009 Nov;103(5):750-4. doi: 10.1093/bja/aep263. PMID: 19797249.
10. Zhang Y, Feng S, Cheng X, Lou K, Liu X, Zhuo M, Chen L, Ye J. The potential value of exosomes as adjuvants for novel biologic local anesthetics. Front Pharmacol. 2023 Jan 26;14:1112743. doi: 10.3389/fphar.2023.1112743. PMID: 36778004; PMCID: PMC9909291.
Table 1. Local anesthetics and adjuvants for nerve blocks. Indicative doses – clinicians need to verify dosing according to local circumstances.
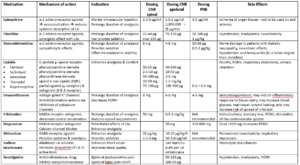
SNRI: serotonin/norepinephrine reuptake inhibitor; LA: local anesthetics; NMDA: N-methyl-D-aspartate; PONV: postoperative nausea and vomiting.